
Rhenium (Re) is a scarce, strategically vital metal with unique properties that make it indispensable in high-temperature superalloys for jet engines and power generation, as well as in platinum-rhenium catalysts for the petrochemical industry. Its limited natural abundance, with an estimated average crustal concentration of just 0.4 parts per billion, and projections of resource depletion within a century, underscore the critical need for efficient recycling from secondary sources.
The demand for rhenium is steadily increasing with technological advancements, yet its substitution in key applications remains challenging without incurring significant performance losses or higher costs. Recycling not only alleviates the pressure on primary resource extraction but also avoids substantial mining costs, waste generation, and associated environmental impacts.
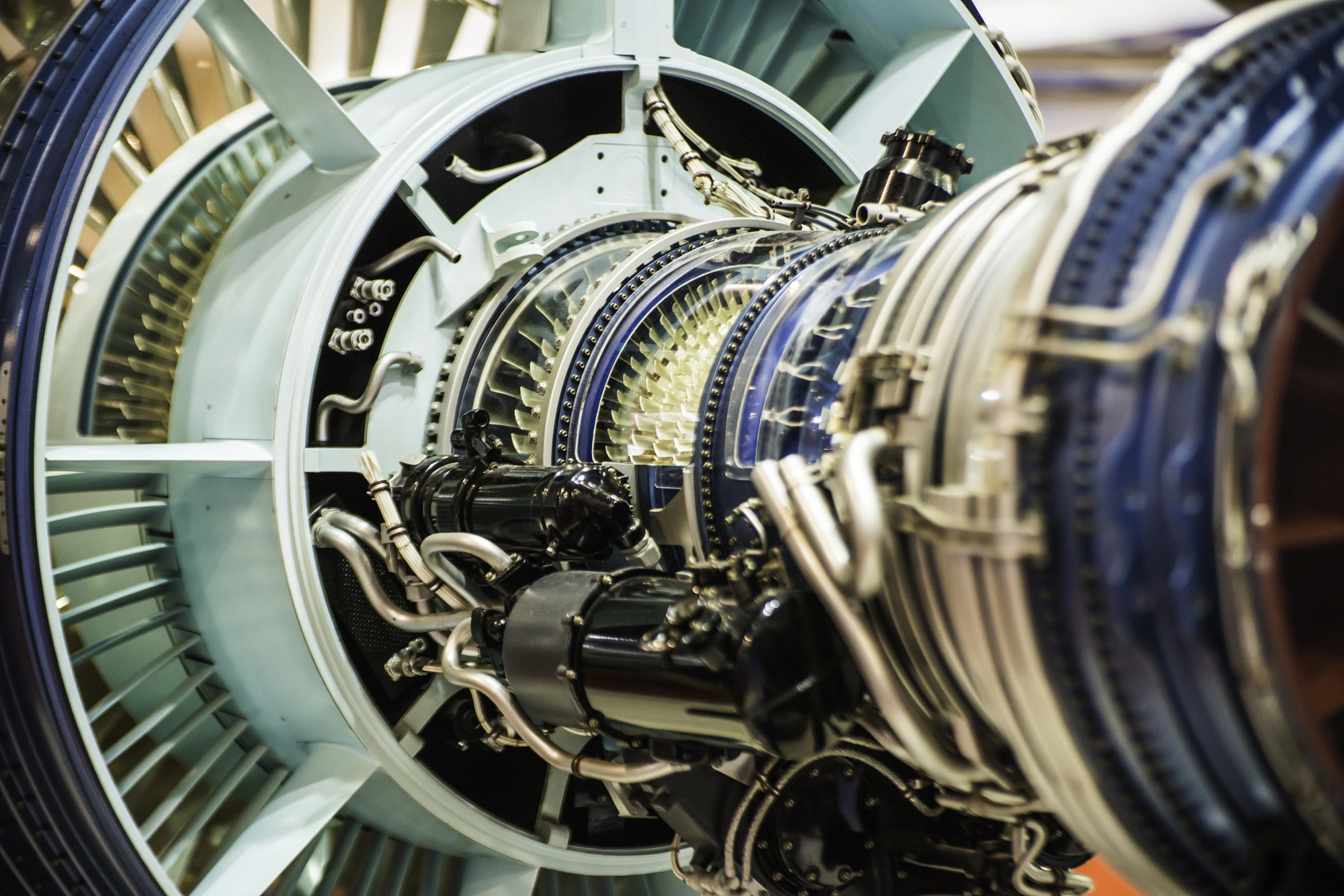
Secondary resources for rhenium generally contain significantly higher concentrations of the metal compared to primary ores. These sources primarily encompass Ni-Re superalloys, which are a major contributor, typically containing 1–20 wt% Re, with common commercial alloys having 3–6 wt% Re; used turbine blades from jet engines serve as a prime example of this category.
Another key secondary resource is W-Re and Mo-Re alloys, which usually contain 3–5 wt% Re and find use in applications such as heating elements and X-ray tubes. Finally, spent Re-containing catalysts, predominantly from the petroleum refining industry, represent a significant stream, typically containing around 0.3 wt% Re, often alongside platinum, on an alumina support. Globally, it’s estimated that at least 10 tons of rhenium are recycled annually from such spent scrap, with a potential ultimate recyclability of over 80%.
Rhenium recycling is a multi-faceted process that typically combines pyrometallurgical (high-temperature processing) and hydrometallurgical (aqueous solution-based processing) techniques. The choice of specific methods is heavily influenced by the composition of the secondary resource and the need to recover other valuable metals present in the scrap. The overarching goal is to efficiently separate rhenium and other metals from the scrap matrix, after which the purification steps often mirror those used in extracting rhenium from primary resources.
Over the years, various methods have been developed for recovering rhenium from superalloy scrap. One notable approach is the electrolytic decomposition method proposed by Stoller et al. in 2008. This process involves low-frequency electrolysis in a cell containing a 15–25 wt% hydrochloric acid solution. Once the electrolytic process is complete, the remaining scrap undergoes an oxidizing leach using a sodium hydroxide-hydrogen peroxide solution. Rhenium is then recovered from the resulting filtrate through ion exchange.
Another technique, described by Olbrich et al. in 2009, uses molten-salt digestion. In this method, superalloys are digested in a molten-salt mixture containing 60–95 wt% sodium hydroxide and 5–40 wt% sodium sulfate at temperatures between 800 and 1200 °C. The digestion process is often enhanced with additives, such as sodium carbonate, and with oxidizing agents, such as sodium nitrate. The end product is subsequently dissolved in water for hydrometallurgical metal separation.
Further advancements were made by Palant et al. between 2011 and 2014, who developed a complex electrochemical process using electrolytes such as sulfuric acid, a mixture of sulfuric and hydrochloric acids, or nitric acid. In this setup, about 70% of the rhenium is recovered from anode slimes, which are then leached with ammonia. In comparison, 25–30% of the transfers to the electrolyte are retrieved through solvent extraction, leading to the production of KReO4 crystals.
Additionally, Dasan et al. introduced a method in 2011 that begins with grinding the scrap into fine particles (~5 µm) to increase the surface area. The ground material is then oxidized, converting rhenium into a volatile oxide form. Following this, Luederitz et al. patented a process in 2013 in which all metals are solubilized using hydrochloric acid or mixtures of hydrochloric and nitric acids. This technique allows selective precipitation of rhenium as Re2S7, which is subsequently oxidized and sublimed to Re2O7.
In 2016, Srivastava et al. devised a two-step leaching process in which base metals such as nickel, aluminum, chromium, cobalt, and tungsten are first leached with 4 mol/L hydrochloric acid. Rhenium remains in the residue and is subsequently leached with in-situ-generated chlorine in the same hydrochloric acid solution before solvent extraction.
Britton and Markarian, in 2017, patented another method involving digestion with a 50–1000 g/L sulfuric acid solution along with a halide-free oxidant, such as air, ozone, oxygen, or peroxide. This is followed by further digestion of the filter cake and the separation of rhenium and platinum through ion exchange.
In a different approach, Kim et al. (2018) explored pyrometallurgical pretreatment followed by leaching. In this process, superalloy scrap with particle size less than 150 μm is pretreated with aluminum granules at approximately 1500 °C, forming Al3Ni. This is followed by a two-step hydrochloric acid leaching: first, extracting nickel, aluminum, cobalt, and chromium; and subsequently, leaching rhenium from the residue using electrogenerated chlorine, leaving tantalum behind.
Finally, Mamo et al. (2019) investigated the use of aqua regia for rhenium recovery, which included a two-stage precipitation process. This process involved precipitating oxides of aluminum, chromium, molybdenum, and titanium at pH 5.05, followed by precipitating mixed hydroxides of cobalt and nickel at pH 7.0, ultimately yielding a rhenium-enriched solution.
Spent catalysts typically contain around 0.3 wt% rhenium (Re) and 0.3 wt% platinum (Pt) on an alumina carrier, and recovery methods often focus on selectively extracting these valuable metals. Before the 1980s, historical caustic dissolution was a common recovery method, but it posed significant challenges for the effective recovery of rhenium.
In more recent practices, sulfuric acid dissolution has emerged as a more favorable approach. This method generally involves leaching rhenium and aluminum with sulfuric acid or sodium hydroxide, often with reducing agents added to retain platinum in the residue. Following this step, rhenium can be separated from the aluminum sulfate solution through ion exchange or solvent extraction, as detailed by El Guindy in 1997.
Another method discussed by Elutin et al. in 1997 is selective roasting and leaching. This technique entails roasting the materials, followed by acid or alkali leaching and ion exchange to produce high-purity ammonium perrhenate (NH4ReO4). Additionally, Han and Meng patented a process in 1996 known as selective pressure leaching. This involves using ammonium halogen salts—specifically iodide or bromide—along with oxygen and sulfuric acid at elevated temperatures and pressures, enabling the recovery of both rhenium and platinum via various methods.
Research by Angelidis et al. in 1999 introduced a simplified dissolution process using dilute sodium bicarbonate. This method allows for up to 97% recovery of rhenium initially, followed by sulfuric acid leaching to extract any remaining platinum and aluminum. Furthermore, Thomas patented a process in 2008 for halogen acid dissolution followed by resin extraction.
This method targets the extraction of rhenium, gold, and platinum-group metals from an acid solution—preferably a combination of a halogen acid and a halogen element—and uses a non-crosslinked polyamine composite resin, along with solvent extraction techniques.
In 2003, Allison et al. patented a unique oxidation and sublimation method. This technique converts rhenium into a sublimable oxide by heating it in an oxidizing atmosphere, enabling its isolation from the resulting volatilized oxide. There are also general acid-leaching techniques that have been applied to the processing of superalloys, as reported by researchers such as Luederitz et al. (2013), Ferron and Seeley (2015), and Britton and Markarian (2017).
Lastly, Kasikov and Petrova in 2008 proposed a categorization of catalyst decomposition methods, distinguishing between those that selectively extract rhenium without decomposing the alumina carrier and those that involve carrier decomposition.
The recycling of rhenium from secondary resources is paramount for establishing a sustainable, low-carbon, and resource-efficient economy. The initial crucial step involves the effective collection of end-of-life products and meticulous classification of different types of rhenium-containing scrap. Based on this classification, a suitable and efficient process flowsheet can be determined. This selection process must thoroughly consider several factors.
Economic feasibility is a primary concern, ensuring the recovery process is cost-effective. Recycle efficiency is also key, aiming to maximize the yield of rhenium and other valuable metals. Environmental factors must be addressed by minimizing waste and harmful emissions. The potential recycling of other metals present in the scrap should be integrated into the process. Finally, the utilization of existing industrial equipment can help to reduce capital investment.
The metallurgy of rhenium, encompassing both primary extraction and secondary recycling, has made significant strides. While primary extraction focuses on enriching rhenium fractions from molybdenum, copper, lead, and uranium processing, recycling efforts are centered on efficiently processing diverse and often complex spent materials.
The continued development and optimization of recycling technologies are essential to ensuring a stable, sustainable supply chain for this critical metal, supporting high-tech industries and emerging innovations.
For organizations looking to responsibly manage their rhenium-containing scrap and contribute to a circular economy, Quest Metals offers state-of-the-art recycling solutions. By partnering with Quest Metals, you can ensure that your rhenium scrap is processed with high efficiency, environmental consideration, and economic viability, transforming waste streams into valuable resources.
Critical evaluations of all recycling processes remain necessary to identify the most sustainable options and build a resilient rhenium industry for the future. Quest Metals is committed to being at the forefront of these advancements.